Service
Product Search

Cyclic Peptides Synthesis
Cyclic peptides are considerably more stable than linear peptides due to their resistance to proteases. They are of great interest to the biotechnology and pharmaceutical industries. Crefu Peptides can synthesis several type of cyclic peptides as below:
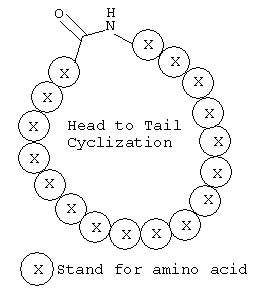
Peptides Head-to-Tail Cyclization
Head to tail peptides are easily assembled via the formation of an amid bond between the peptides own N-terminus amine and C-terminus carboxylic acid. We perform the reaction with very high yields, and routinely provide over 98% purified cycled peptides.
Cyclic peptides are considerably more stable than linear peptides due to their resistance to proteases. They are of great interest to the biotechnology and pharmaceutical industries. Crefu Peptides can synthesis several type of cyclic peptides as below:
Peptides Head-to-Tail Cyclization
Head to tail peptides are easily assembled via the formation of an amid bond between the peptides own N-terminus amine and C-terminus carboxylic acid. We perform the reaction with very high yields, and routinely provide over 98% purified cycled peptides.
 |
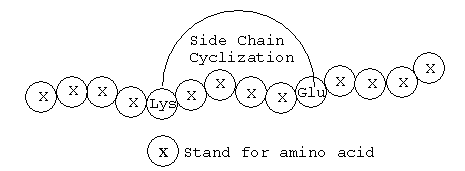
Peptides Side Chain Cyclization
Crefu Peptides have the capable to selectively protect, and then deprotect peptide sidechains to allow for the formation of lactam bridges between Lysine or Diaminopropanoic acid, and Glutamic and Aspartic Acids. We can even perform multiple different sites for bridge formation.
Crefu Peptides have the capable to selectively protect, and then deprotect peptide sidechains to allow for the formation of lactam bridges between Lysine or Diaminopropanoic acid, and Glutamic and Aspartic Acids. We can even perform multiple different sites for bridge formation.
 |
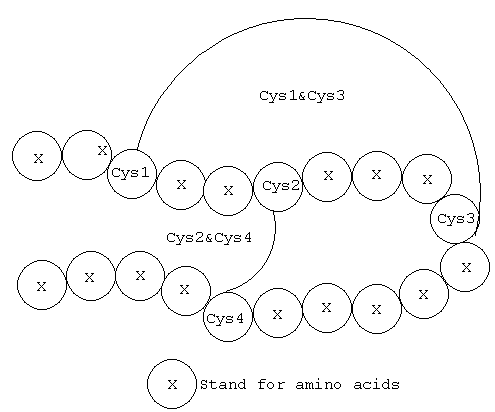
Cys-Cys Disulfide Bridge Cyclization
Disulfide-rich cyclic peptides have generated great interest in the development of peptide-based therapeutics due to their exceptional stability toward chemical, enzymatic, or thermal attack. We routinely synthesize single, double and multiple disulfide bridges on peptides with very high purity yields.
Disulfide-rich cyclic peptides have generated great interest in the development of peptide-based therapeutics due to their exceptional stability toward chemical, enzymatic, or thermal attack. We routinely synthesize single, double and multiple disulfide bridges on peptides with very high purity yields.
 |
Custom Hydrocarbon Stapled Peptides Synthesis
Hydrocarbon-stapled peptides are designed to mimic an α-helical structure through a constraint imposed by covalently linking two residues on the same helical face (e.g., residue i with i + 4; i and i+7).
Hydrocarbon-stapled peptides are designed to mimic an α-helical structure through a constraint imposed by covalently linking two residues on the same helical face (e.g., residue i with i + 4; i and i+7).
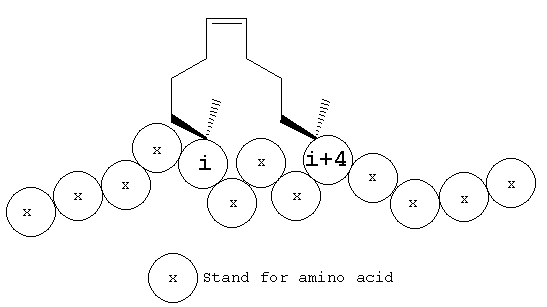 |
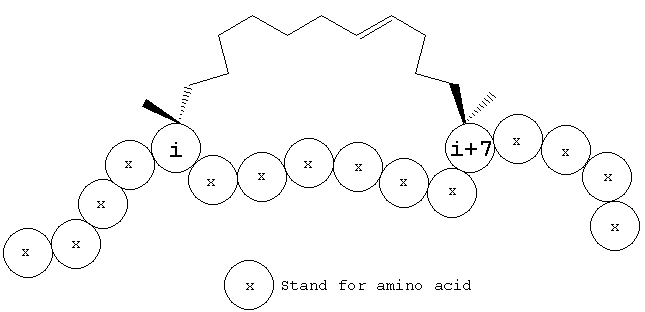 |